

In recent years, probiotics have become the subject of extensive research. This attention is mainly due to these microorganisms’ essential beneficial action on human health. Biofarma Group recognizes this importance and has been active in the formulation and production of probiotic-based drugs for years. To study and predict their effects, however, it is necessary to be able to quantify them as accurately as possible.
That is why a team of researchers composed of Luca Michelutti and Emanuele Nencioni of Biofarma Group, with the support of Dr. Michela Bulfoni of the Institute of Pathological Anatomy of the ASU FC of Udine, have carried out a study on the search for bacterial probiotics through a new method called flow cytometry.
First, it is necessary to clarify what probiotics are and what they are used for.
Probiotics are “good bacteria” that, resisting the action of gastric juice and bile secretion, can settle in the intestine and thus counteract the adhesion of some pathogenic germs. Their presence promotes a balance of the intestinal bacterial flora and positively influences the immune system while reducing blood cholesterol levels. In addition, it improves food allergy problems and helps in the case of diverticulosis, irritable bowel syndrome, and urinary tract infections.
Their importance is also recognized in many fields: pharmaceutical, food, water, and the environment.
In the pharmaceutical field, in particular, knowing how to quantify the number of vital probiotics within a heterogeneous population is fundamental as it allows understanding of the level of effectiveness of the product on the human body.
A key feature of probiotics is that they are metabolically active to proliferate and exert their beneficial effects within the small intestine and colon. The active state, however, is not detectable by a traditional measurement method since the latter only provides information on microbiotic growth and the ability of microorganisms to duplicate in vitro. On the other hand, damaged and quiescent cells cannot be detected correctly, which can cause quantification errors.
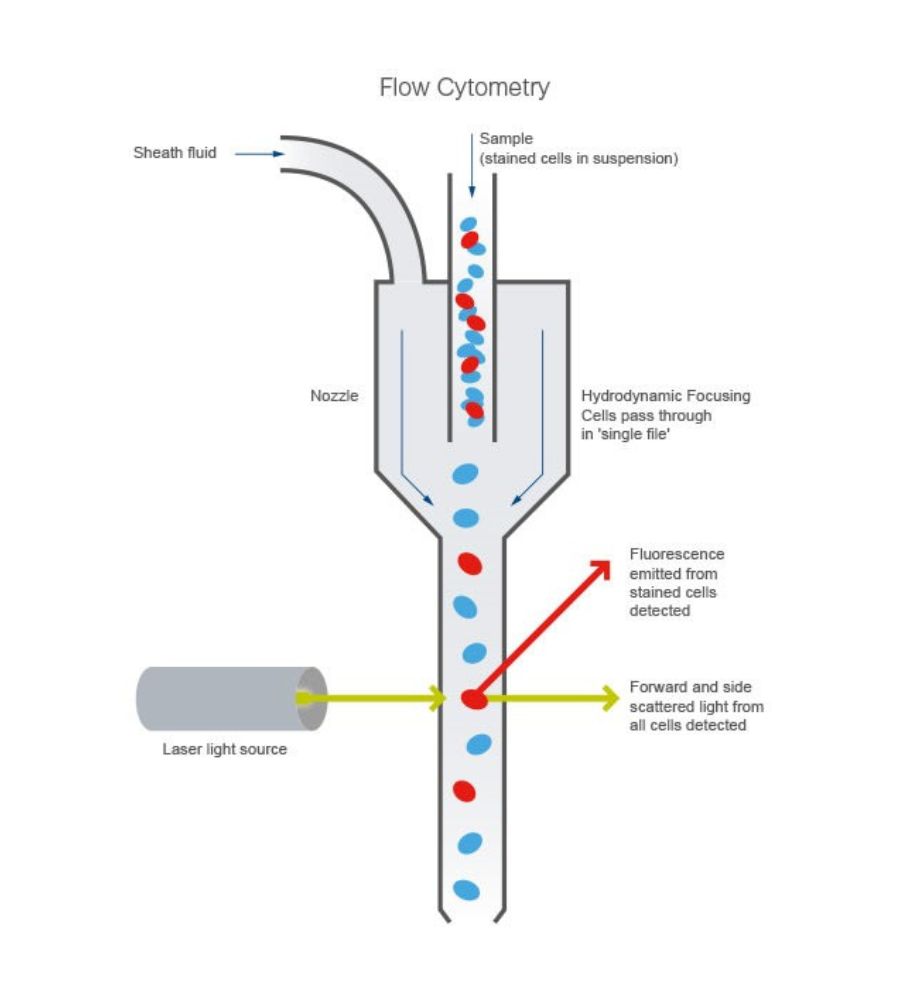
The solution that allows overcoming this measurement problem is flow cytometry. It is a technique that helps analyze, quantify and separate cells or microparticles. In the specific case of probiotics, it can measure vitality and efficiency, analyzing metabolic activity, fermentation capacity, acidification potential, and oxygen absorption capacity.
The cytofluorimetric technique includes different phases. Starting from a sample of cells suspended in a fluid and treated with specific dyes (fluorochrome) able to distinguish cellular subtypes. It is then inserted into the instrument called, precisely, cytofluorimeter. It is channeled into the flow chamber and, subsequently, through a very narrow hole, the cells continue in an orderly manner inside the tube. The flow, therefore, is intercepted by different detectors that analyze all the cells inside at high speed, identifying their key characteristics such as, for example, the size or cell complexity. The signal intercepted by the various detectors is amplified and sent to the computer, which converts the result into a digital file, usually in the form of graphs, sent to print.
Biofarma Group conducted a study to evaluate the advantages deriving from the use of an analytical method based on the cytometric flow approach.
This analysis is not usually applied in microbiological examinations, where the most widespread tool for routine bacterial enumeration remains plate counting (Gracias & McKillip, 2004; Holm et al., 2004; Jozwa and Czaczyk, 2012).
Specifically, the researchers’ goal was to apply ICH parameters, generally used for the analysis of drugs and chemical compounds on probiotic-based products, thus comparing the classic method with the new vitality test.
Almost all statistical values confirmed equivalent effectiveness between the two methods compared. The results obtained from the finished products and the reconstituted samples indicated a good correlation between plate counts and flow cytometry; moreover, the data relating to the second seemed to be replicable with greater precision and solidity.
Considering all the results, the optimization and validation of the flow cytometry technique in compliance with the ICH recommendations has laid the foundations for applying chemical-pharmaceutical rules. Also, the count of probiotics allows the identification of viable and non-viable cells and provides additional information on their physiological state and metabolic activity.
This policy also has further advantages: in addition to lower costs, a significant reduction in the analysis time has been observed, i.e., 2 hours compared to the three days required by the traditional method, or also to the fact that it is possible to analyze and quantify thousands of active cells even in a tiny sample.
Finally, applying a chemical-pharmaceutical validation approach, using ICH on vital probiotics, ensures greater quality control in flow cytometry than the method normally used.