

Mereto, located in the North-East of Italy in the province of Udine, is the Group's headquarters and production site of excellence, guaranteeing a reliable and tailored manufacturing service in production facilities that comply with the latest international GMP guidelines and ISO 9001 and 14001. In addition to periodic audits by the Italian Medicines Agency (AIFA), the Mereto production site is regularly inspected by numerous Health Authorities and many multinationals to which we provide contract manufacturing services.
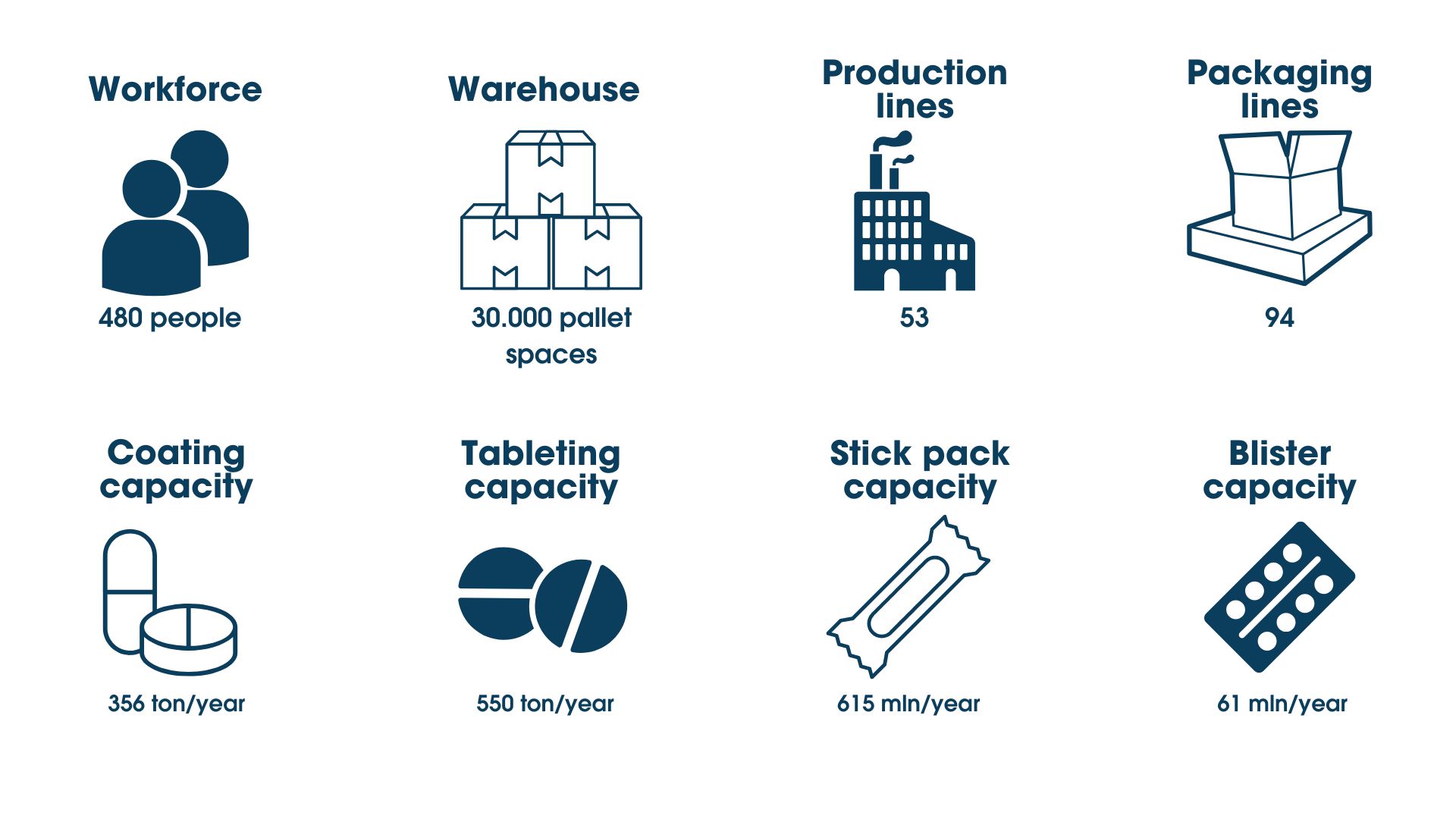
The Biofarma Group site in Mereto boasts state-of-the-art production facilities, specialising in the production of nutraceuticals in solid and liquid form, medical devices for topical and oral use, sports supplements, and cosmetics, covering a total area of 44,000 m2.
With a range of production capabilities covering powders, coated and uncoated tablets, as well as hard capsules, the plant of Mereto can deliver on almost every oral solid dosage form and drive the customer’s project to success.

In particular, the Biofarma Group plant of Mereto di Tomba can handle projects on several pharmaceutical forms:
Tablets
Chewable Tablets
Capsules
Powder
ODT’s tablets
Mereto plant can support all the semi-solid development project needs. We have a wealth of experience tackling the challenges associated with the development of semi-solids, from managing chemical stability, to physical stability and dose homogeneity.

We produce a wide range of topical and oral dosage forms, including:
Emulsions
Gels
Ointments, butters, scrubs and muds
Creams and pastes
We have a strong experience in overcoming the challenges associated with liquid formulation development. Our team of experts is adept at managing the chemical stability requirements of liquid products, as well as physical stability and dose homogeneity.

In particular, the liquid formats covered by the plant are:
Water-based products
Oil-based products
Alcoholic solutions
Oils sprays
Multi-phasic
In the Mereto di Tomba plant, there is an area authorized and certified by the Italian Medicines Agency (AIFA) dedicated to the production for the world market - in the same environments and plants - of drugs and food supplements based on lactic ferments.

The products are made in a 3,500 square meters site (1,000 are external). It houses 15 production rooms distributed over three floors, in strict environmental conditions in ISO8 class, with temperatures and humidity strictly controlled to ensure best-in-class quality standards and avoid microbial contamination. This division results from constant investments in cutting-edge production processes and solid collaborations, which are fundamental to developing new ideas and creating quality products and competitive solutions on the market.
The Biofarma Group plant of Mereto di Tomba produces two exclusive, patented multi-phase technologies: Dry-Cap and M-Cap.

They are based on a special two-phase packaging technology making it possible to enclose and at the same time separate in the same main pack, the solid component (powder) and liquid component of the product. This type of packaging is particularly well-suited to probiotic-based drugs, but it can also be used for vitamins, minerals and other micronutrients.
Whatever your product, it is essential to ensure it reaches the global market efficiently and on time. Packaging supply directly impacts time-to-market. At Mereto Plant, we specialise in a range of cosmetic and nutraceutical packaging services tailored to customer’s unique products, ensuring speed to market.

Our array of packaging includes:
Blister packaging (ALU-ALU, ALU-PVC)
Bottle and vial packaging
Pill jars
Ampoule packaging
Tubes
Sachets and stick packs
Roll-on
In the Mereto di Tomba plant there is an industrial facility called Biofarma2. From 2020, this facility houses the new packaging department for topical products on an area of 3,800 square metres entirely designed in lean manufacturing. In bodies B and C of the new complex, on the other hand, the Logistics Centre will be housed from July 2019: consisting of a logistics area and a highly computerised and automated shipping area, it can accommodate up to 20,205 pallet spaces.
Food Supplements liquids
Food Supplement solids
Medical Devices
Cosmetics
Probiotics
Via Castelliere 2, 33036 Mereto di Tomba (UD) - Italia
Tel.: +39 0432 868711
E-mail: marketing@biofarmagroup.it
